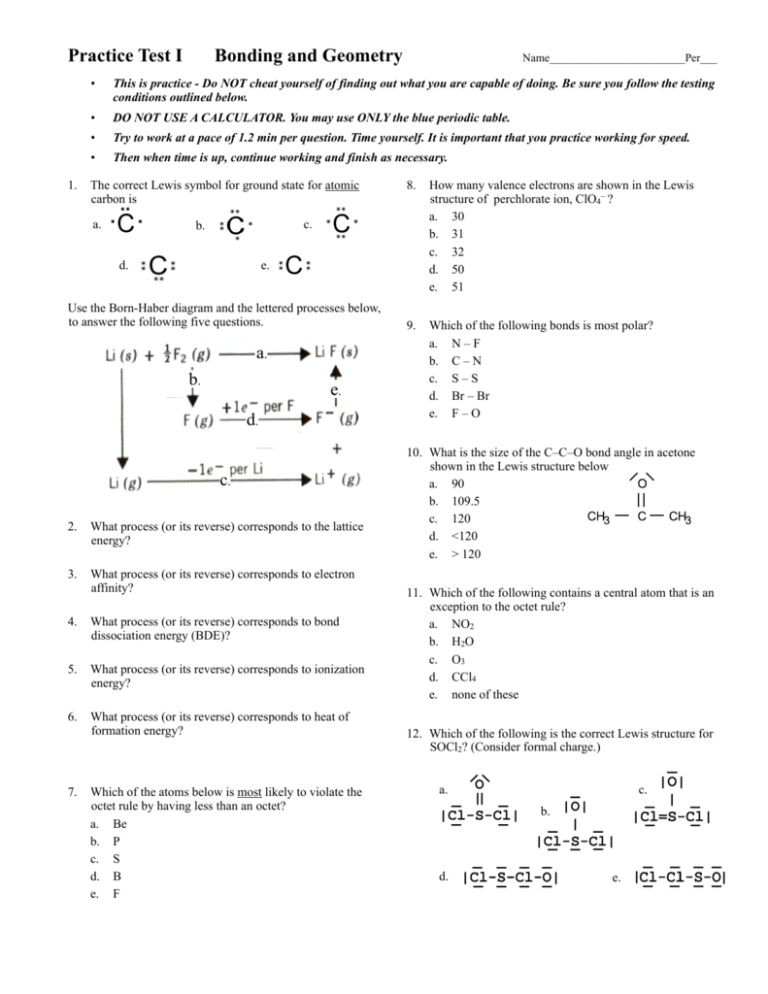
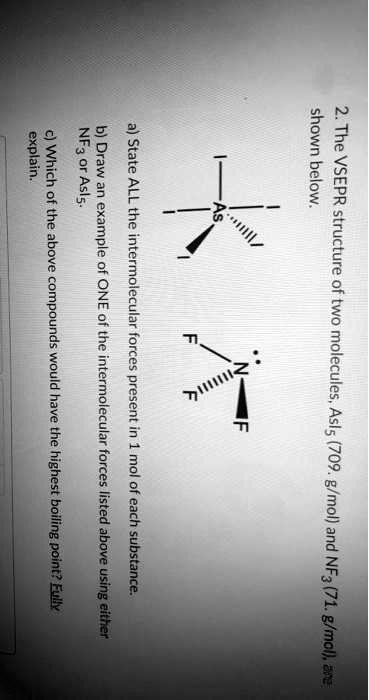
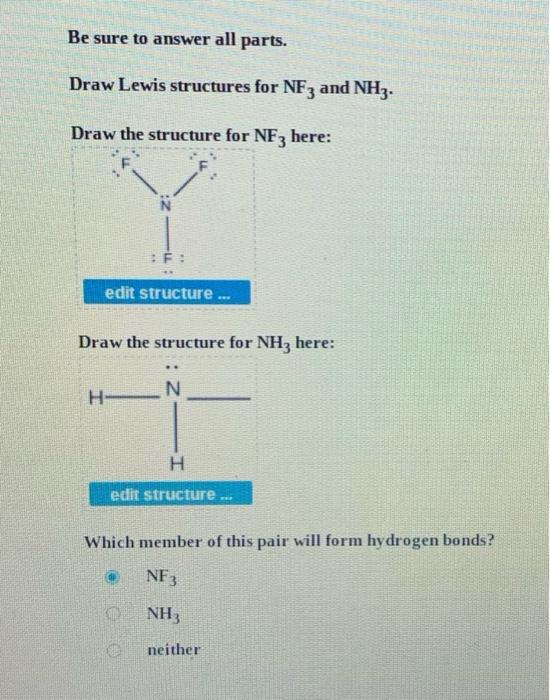
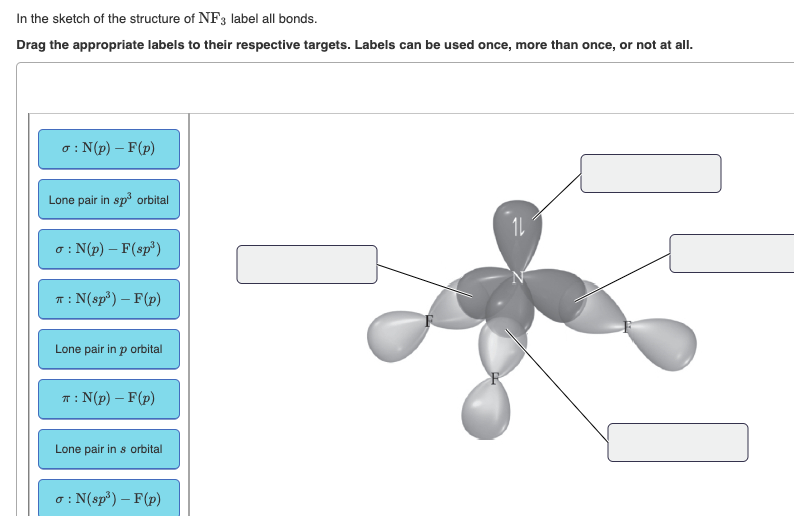
41 in the sketch of the structure of nf3 label all bonds.
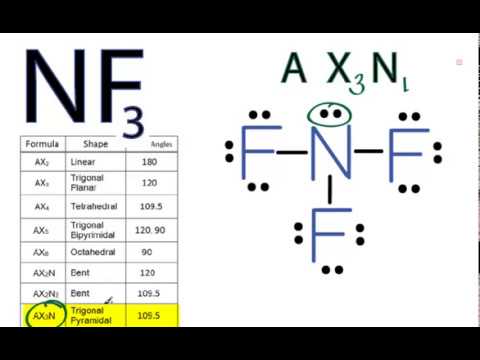
Ch2br2 bonds - kgoj.visavietnam.pl While the molecule contains polar bonds (C - Br), the overall molecule is nonpolar due to there being an overall net dipole of zero A molecule's 3-d geometry explains a lot of its physical and chemical properties, like its polarity and intermolecular bonding behavior Drawing the Lewis Structure for C 2 H 2 (Ethyne or Acetylene) Log Octanol. NF3 lewis structure, molecular geometry, bond angle ... - Topblogtenz Simple steps for drawing the NF3 lewis dot structure 1. Count total valence electron in NF3 In the first step, we have to calculate the total number of valence electrons present in the NF3 molecule. Nitrogen is present in the 15th group in the periodic table and Fluorine in group 17th. ⇒ Total valence electron in Nitrogen = 5
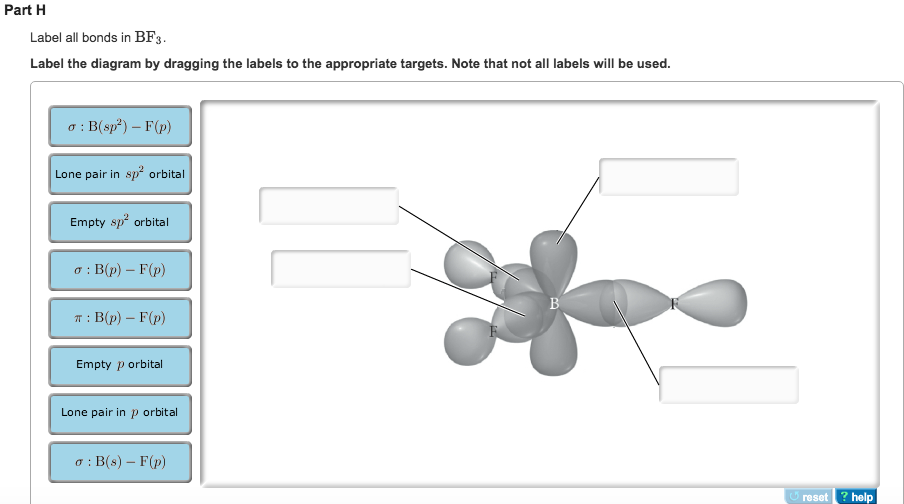
Answered: In the sketch of the structure of BF3… | bartleby Transcribed Image Text: In the sketch of the structure of BF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Lone pair in sp orbital : B(p) - F(p) Empty p orbital Lone pair in p orbital B B(sp²) - F(p) в : В(8) — F(p) o : B(p) - F(p) Empty sp? orbital

In the sketch of the structure of nf3 label all bonds.
Answered: 1. Rank the bonds below from most… | bartleby 1. Rank the bonds below from most reactive (1) to lcast reactive (4) Cl-CI Br-Br F-F 2. Explain your reasoning for the rankings in #1. Draw a picture to explain as well! Answered: In the sketch of the structure of NF3… | bartleby Chemistry Q&A Library In the sketch of the structure of NF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. σ: Νip) - F(p) Lone pair in sp orbital 1L σ: Nip) - F (sp') T:N(sp³) - F(p) Lone pair in p orbital T: N(p) - F(p) Lone pair in s orbital σ: Ν(sp ... Solved Label all bonds in BF3. Label the diagram by dragging - Chegg Expert Answer. 96% (26 ratings) Transcribed image text: Label all bonds in BF3. Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. σ : B (s)-F (p) Empty p orbital Lone pair in sp orbital σ : B (sp2)-F (p) σ : B (p)-F (p) Lone pair in p orbital Empty sp2 orbital π : B (p)-F (p ...
In the sketch of the structure of nf3 label all bonds.. Solved In the sketch of the structure of NF3 label all | Chegg.com Transcribed image text: In the sketch of the structure of NF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. SOLVED:Write a hybridization and bonding scheme for each ... - Numerade We drew two bonds, um, or two bonds to each oxygen, which takes four of its electrons, and it has a total of six. So we have an extra lone electron pair here. So adding up the total things on the sulfur we see that's using to pure metals, Um, and two Sigma or Bills and the lone electron pairs. So this has to be S P three d. Solved In the sketch of the structure of BF3 label all | Chegg.com Expert Answer 100% (32 ratings) Transcribed image text: In the sketch of the structure of BF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Condensed structural formula for given compounds has to be given ... The structure of a molecule can be drawn by analyzing the presence of prefix, suffix and root word in the given IUPAC name. Condensed structural formula is representation of the organic compound. In this the lengthy carbon chain is shown only with the carbon atoms (along with the hydrogen) without any bonds.
NCl3 lewis structure, molecular geometry, bond angle ... - Topblogtenz NF3 lewis structure has 3 fluorine and 1 nitrogen atom connected with three single bonds and NH3 lewis structure has 3 hydrogens and 1 nitrogen connected with three single bonds also. In all these molecules(NH3, NF3, and NCl3), there is one lone pair present on the central atom. (Get Answer) - Label all bonds in CH2Br2. Label all bonds ... - Transtutors Label all bonds in NF3. Label all bonds in BF3. Label all bonds in CH2Br2 Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. o C (sp) H (s) o C (sp') Br (s) C (p) H (p) C (p) Br (p) C (sp) H (p) o C (sps) Br (p) C (sps) Br (p) reset help Expert's Answer Solution.pdf Next Previous Q: BF3 Lewis Structure, Molecular Geometry, Hybridization, and Polarity To draw a Lewis Structure, first of all, add electrons and draw the connectivities. As discussed, here there are 24 electrons. Then, add octets to the outer atom and extra electrons to the central atom. But, as we know, there are no extra electrons. (24 - 24 = 0) Violations Label all bonds in CH2Br2. Label all bonds in SO2. Label all bonds in ... Label all bonds in NF3. Label all bonds in BF3. Answer The molecule's name is CH2Br Br Br br dibromomethane. The molecule can be described as a derivative methane. The central atom of carbon is bonded with two hydrogen atoms, and two bromine. All bonds are sigma. Below is the electron configuration for the atoms.
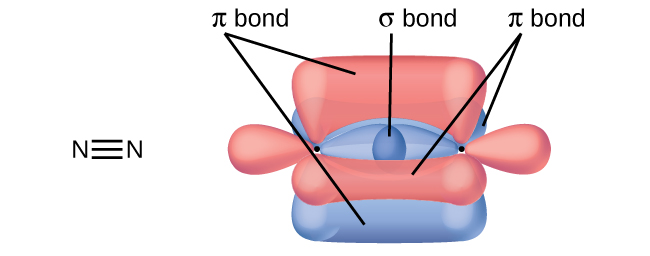
NBr3 lewis structure, molecular geometry, bond angle ... - Topblogtenz Nitrogen tribromide (NBr3) lewis dot structure, molecular geometry, polar or non-polar, hybridization. Nitrogen tribromide appears as deep red is a chemical compound having the molecular formula NBr3. In pure form, it is very explosive in nature. It is highly unstable and can be hydrolyzed in water. In this article, we will discuss NBr3 lewis ... Chapter 6, Chemical Bonding II Video Solutions, Chemistry: Structure ... Write a hybridization and bonding scheme for each molecule that contains more than one interior atom. Indicate the hybridization about each interior atom. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. C2H2 (skeletal structure HCCH) b. Answered: The structure of acetylsalicylic acid… | bartleby The structure of acetylsalicylic acid (aspirin) is shown here. How many pi bonds are present in acetylsalicylic acid? How many sigma bonds? Which parts of the molecule are free to rotate? SOLVED:Write a hybridization and bonding scheme for COCl2 ... - Numerade Answer Write a hybridization and bonding scheme for each molecule. Sketch the molecule, including overlapping orbitals, and label all bonds using the notation shown in Examples 10.6 and 10.7. a. C C l 4 b. N H 3 c. O F 2 d. C O 2 Discussion You must be signed in to discuss. Video Transcript So we're continuing on with molecular bonding.
Use valence bond theory to write the hybridization and ... - Socratic Step 1. Draw the Lewis structure (a) Start with a skeleton structure. The two #"C"# atoms (least electronegative) will be the central atoms, with the #"N"# attached to one of the carbons. (b) Attach the hydrogen atoms. The question gives you a clue where they go. The formula #"NCCH"_3# tells you that the three #"H"# atoms are attached to the terminal carbon atom.
NH3 Lewis Structure, Geometry, and Hybridization - Techiescientist The molecular geometry of ammonia (NH3) is trigonal pyramidal or a distorted tetrahedral. It is because of the presence of a single lone pair of electrons on the nitrogen atom which is non-bonding in nature and exerts repulsion on the bonding orbitals. If you notice, most of the non-bonding, lone pair of electrons are present on the apex.
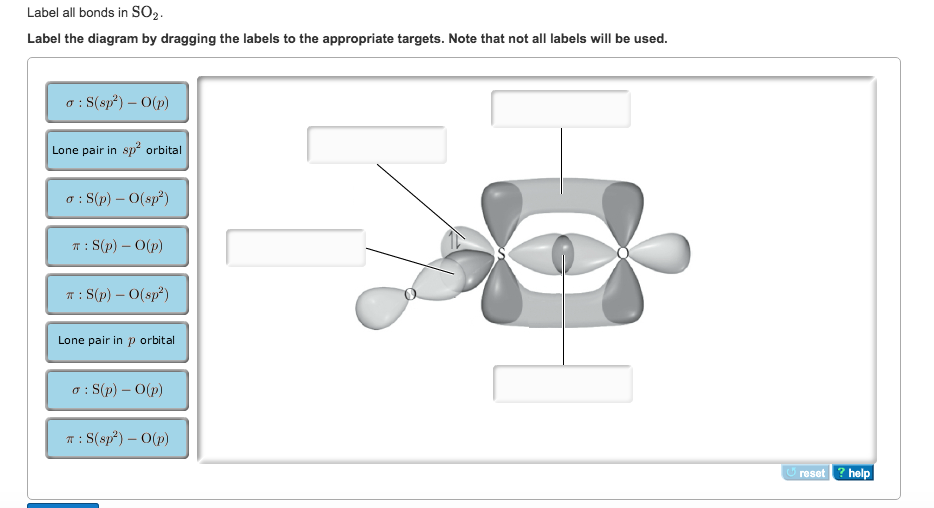
Answered: In the sketch of the structure of SO2… | bartleby In the sketch of the structure of SO2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. : S (p) - O (p) Lone pair in p orbital Lone pair in sp? orbital o : S (p) - O (sp²) т: S (p) — О (p) т: S (sp?)
SOLVED:Write a hybridization and bonding scheme for each ... - Numerade Write a hybridization and bonding scheme for each molecule or ion. Sketch the structure, including overlapping orbitals, and label all bonds using the notation shown in Examples 6.1 and 6.2. a. COCl2 (carbon is the central atom) b. BrF5 c. XeF2 d. I3
Ch2br2 bonds - ezzfq.zakupywnecie.pl Transcribed Image Text: In the sketch of the structure of CH2Br2 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. ... Heterolytic bond breaking is also known as heterolysis or heterolytic fission or ionic fission. It is defined as breaking of a covalent bond.
NH3 Lewis Structure, Molecular Geometry, Hybridization, Bond Angle & Shape NH3 Bond angles There are three single bonds and one lone pair of electrons in NH3 molecule. It has a molecular geometry of trigonal pyramidal which also looks like a distorted tetrahedral structure. The shape is distorted because of the lone pairs of electrons. This pair exerts repulsive forces on the bonding pairs of electrons.
CHEM: Chapter 10 Flashcards | Quizlet The skeletal structures of several simple amino acids are shown here. For each skeletal structure, complete the Lewis structure, determine the geometry and hybridization about each interior atom, and make a sketch of the molecule, using the bond conventions of Section 10.4. (On Doc) Cumulative Problem 92.
Solved Label all bonds in BF3. Label the diagram by dragging - Chegg Expert Answer. 96% (26 ratings) Transcribed image text: Label all bonds in BF3. Label the diagram by dragging the labels to the appropriate targets. Note that not all labels will be used. σ : B (s)-F (p) Empty p orbital Lone pair in sp orbital σ : B (sp2)-F (p) σ : B (p)-F (p) Lone pair in p orbital Empty sp2 orbital π : B (p)-F (p ...
Answered: In the sketch of the structure of NF3… | bartleby Chemistry Q&A Library In the sketch of the structure of NF3 label all bonds. Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. σ: Νip) - F(p) Lone pair in sp orbital 1L σ: Nip) - F (sp') T:N(sp³) - F(p) Lone pair in p orbital T: N(p) - F(p) Lone pair in s orbital σ: Ν(sp ...
Answered: 1. Rank the bonds below from most… | bartleby 1. Rank the bonds below from most reactive (1) to lcast reactive (4) Cl-CI Br-Br F-F 2. Explain your reasoning for the rankings in #1. Draw a picture to explain as well!















![3 - [Catherine-Housecroft, -Alan-G.-Sharpe]-Inorganic-C ...](https://static.docsity.com/documents_first_pages/notas/2017/09/07/a7a7f933ea27716d2544545013164316.png)






Post a Comment for "41 in the sketch of the structure of nf3 label all bonds."