41 open-label study bias
Login to your account - The Lancet Respiratory Medicine 27.06.2022 · Increased neutralising antibody capacity was previously reported in older patients with immune-mediated inflammatory diseases that had changed their methotrexate schedule and extended the dosing interval by more than 7 days at the time of their first or second COVID-19 vaccine dose in a non-randomised study with several limitations: potential recall bias, … Reducing bias in open-label trials where blinded outcome ... by BC Kahan · 2014 · Cited by 74 — Many trial designs do not permit blinding, and are therefore designed as open-label, with patients, clinicians, and other study investigators ...
External and internal validity of open label or double‚•'blind ... by J BEYER‐WESTENDORF · 2011 · Cited by 61 — subject to bias than an open trial because it minimizes the impact of knowledge of treatment ... AF using an open-label study [1] and in acute deep vein.

Open-label study bias
Radiation doses and fractionation schedules in non-low-risk ductal ... In patients with resected non-low-risk DCIS, a tumour bed boost after WBI reduced local recurrence with an increase in grade 2 or greater toxicity. The results provide the first randomised trial data to support the use of boost radiation after postoperative WBI in these patients to improve local control. The international scale of the study supports the generalisability of the results. Investigating Potential Bias in Patient-Reported Outcomes in ... by JK Roydhouse · 2019 · Cited by 29 — This Viewpoint highlights the importance of potential bias in patient-reported outcomes in open-label cancer trials and points to areas of ... Long-term efficacy and safety of moderate-intensity statin with ... 30.07.2022 · Our study has several limitations. First, this was an open-label trial. Physicians and the patients were aware of group assignment, which could potentially have led to bias in reporting patient symptoms. The nocebo effect of the statin therapy should be considered.
Open-label study bias. Transcatheter Mitral-Valve Repair in Patients with Heart Failure Nonetheless, potential bias cannot be completely ruled out. Second, the median follow-up was longer in the device group than in the control group, in part because of the lower mortality in the ... At-home, sublingual ketamine telehealth is a safe and effective ... While this study demonstrates the promising potential of KAT for treating anxiety and depression and using telehealth for overcoming treatment access barriers and ensuring safety through remote monitoring, there are limitations of this study. The first is the drop in symptom survey completions at week 4 for around half of the records reviewed. Bias was reduced in an open-label trial through the removal of ... by BC Kahan · 2016 · Cited by 10 — A recent review found that only 26% of open-label trials reported using blinded outcome assessment [20]. Unblinded outcome assessment can lead to biased ... Bias for Patient-Reported Outcomes in Open-Label Cancer ... 27 Feb 2019 — A common concern with patient-reported outcomes (PROs) in open-label trials is that a patient's knowledge of treatment received could influence ...
Medical Cannabis for the Treatment of Dementia: A Review of … 17.07.2019 · One open-label prospective cohort study included in the SR found that among 11 patients with Alzheimer’s disease, there were statistically significant improvements in the NPI subscales assessed from baseline following 28 days treatment with medical cannabis oil containing THC given orally at an initial dose of 2.5 mg twice daily and titrated up to 7.5 mg … Open-Label, Randomized, Multicenter, Phase III Study Comparing … 20.07.2022 · A phase III open-label study comparing oral paclitaxel plus E (oPac + E) 205 mg/m 2 paclitaxel plus 15 mg E methanesulfonate monohydrate 3 consecutive days per week versus IVpac 175 mg/m 2 once every 3 weeks was performed. Women with metastatic breast cancer and adequate organ function, at least 1 year from last taxane, were randomly assigned 2:1 to oPac … Survivorship bias - Wikipedia Susan Mumm has described how survival bias leads historians to study organisations that are still in existence more than those that have closed. This means large, successful organisations such as the Women's Institute , which were well organised and still have accessible archives for historians to work from, are studied more than smaller charitable organisations, even though … What is an open label trial? | The BMJ 23.05.2014 · Researchers assessed the effectiveness of prazosin combined with scorpion antivenom in assisting recovery from scorpion sting. An open label randomised controlled trial study design was used. The control treatment was prazosin alone. The setting was a hospital and research centre in Mahad, a region of India. Participants were patients with grade 2 scorpion …
Open-label trial - Wikipedia An open-label trial may still be randomized. Open-label trials may also be uncontrolled (without a placebo group), with all participants receiving the same ... GRADE: Pfizer-BioNTech, Moderna, and Janssen COVID-19 … 29.10.2021 · For the Moderna mRNA-1273 booster, two records screened and deemed eligible for full-text review were included in the GRADE evidence synthesis, and additional data was obtained from the study sponsor (Appendix 1). 11–13 Prevention of symptomatic laboratory-confirmed COVID-19 was assessed by immunobridging using open-label Phase II clinical trial data … Reducing bias in open-label trials where blinded ... - NCBI by BC Kahan · 2014 · Cited by 74 — We describe two randomised trials where blinded outcome assessment was not possible, and discuss the strategies used to reduce the possibility ... Long-term efficacy and safety of moderate-intensity statin with ... 30.07.2022 · Our study has several limitations. First, this was an open-label trial. Physicians and the patients were aware of group assignment, which could potentially have led to bias in reporting patient symptoms. The nocebo effect of the statin therapy should be considered.
Investigating Potential Bias in Patient-Reported Outcomes in ... by JK Roydhouse · 2019 · Cited by 29 — This Viewpoint highlights the importance of potential bias in patient-reported outcomes in open-label cancer trials and points to areas of ...
Radiation doses and fractionation schedules in non-low-risk ductal ... In patients with resected non-low-risk DCIS, a tumour bed boost after WBI reduced local recurrence with an increase in grade 2 or greater toxicity. The results provide the first randomised trial data to support the use of boost radiation after postoperative WBI in these patients to improve local control. The international scale of the study supports the generalisability of the results.






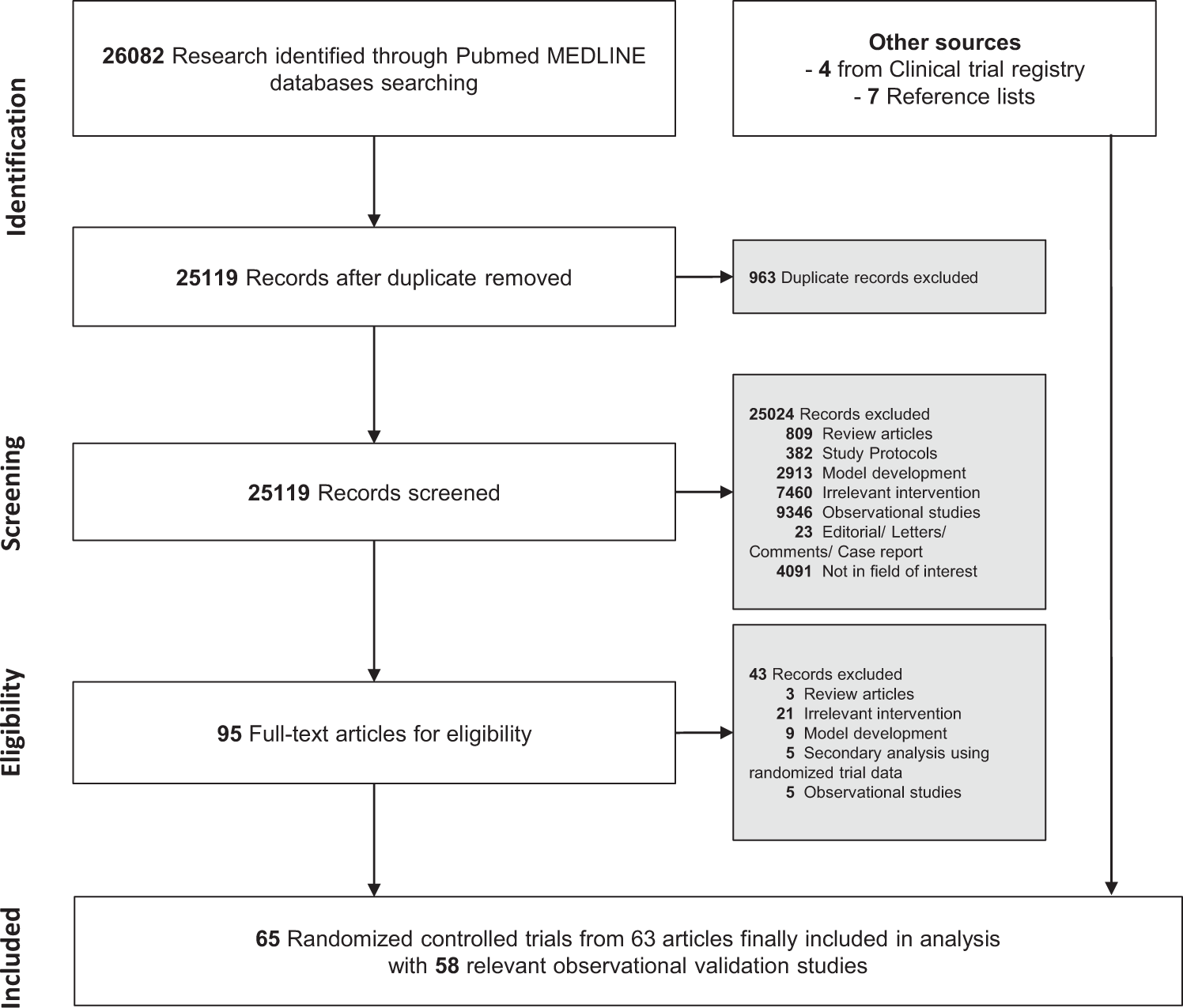











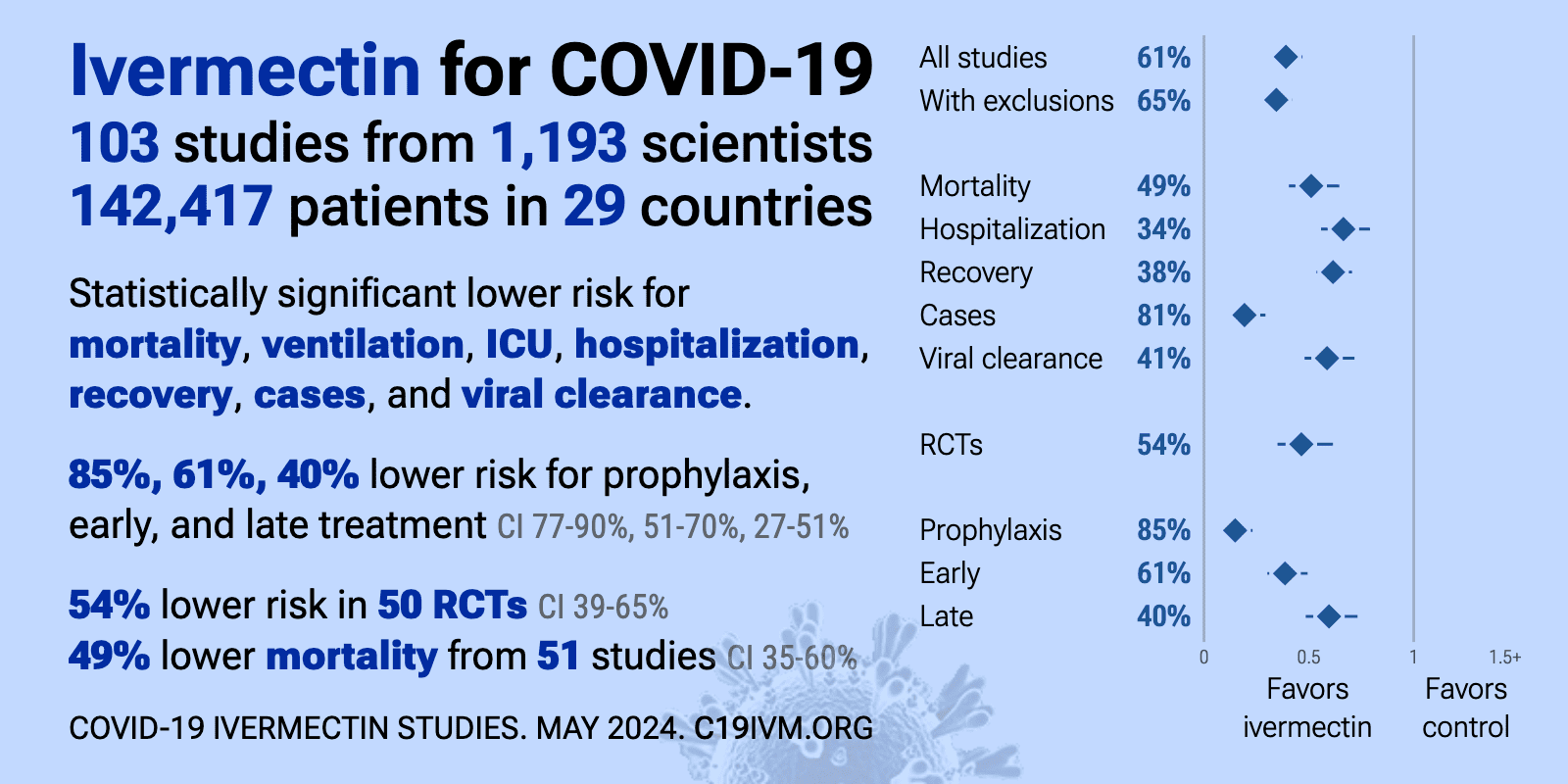

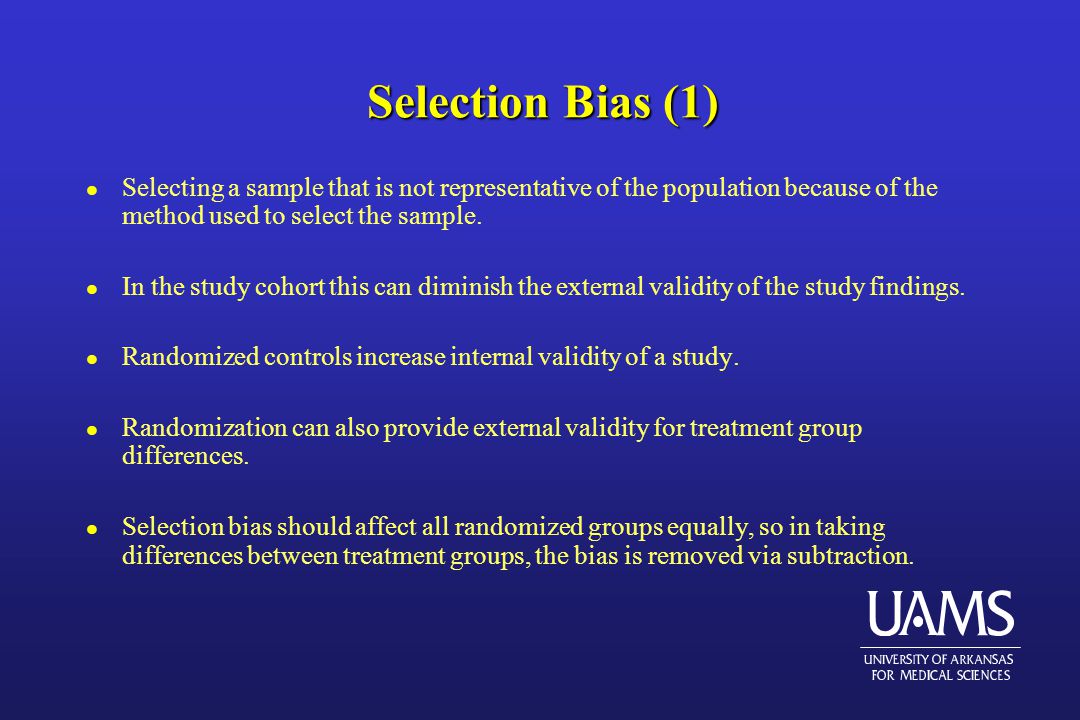



![PDF] Bias was reduced in an open-label trial through the ...](https://d3i71xaburhd42.cloudfront.net/814ad6ccbfa9defca4d2b00c4672f9070cf6b8da/16-Figure1-1.png)








Post a Comment for "41 open-label study bias"