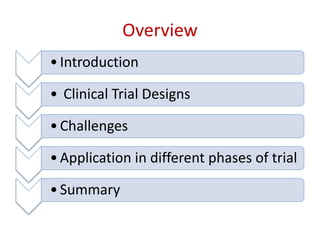
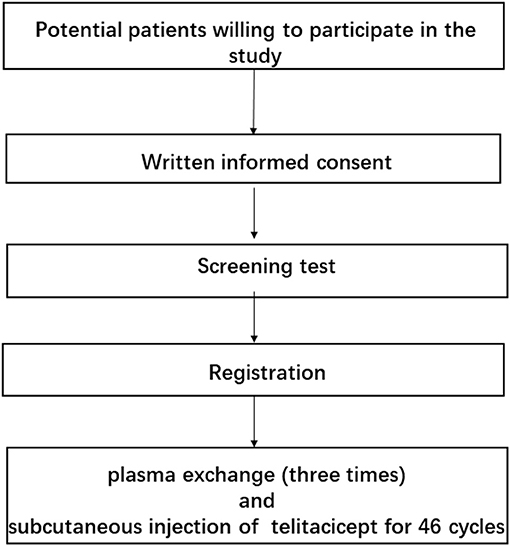
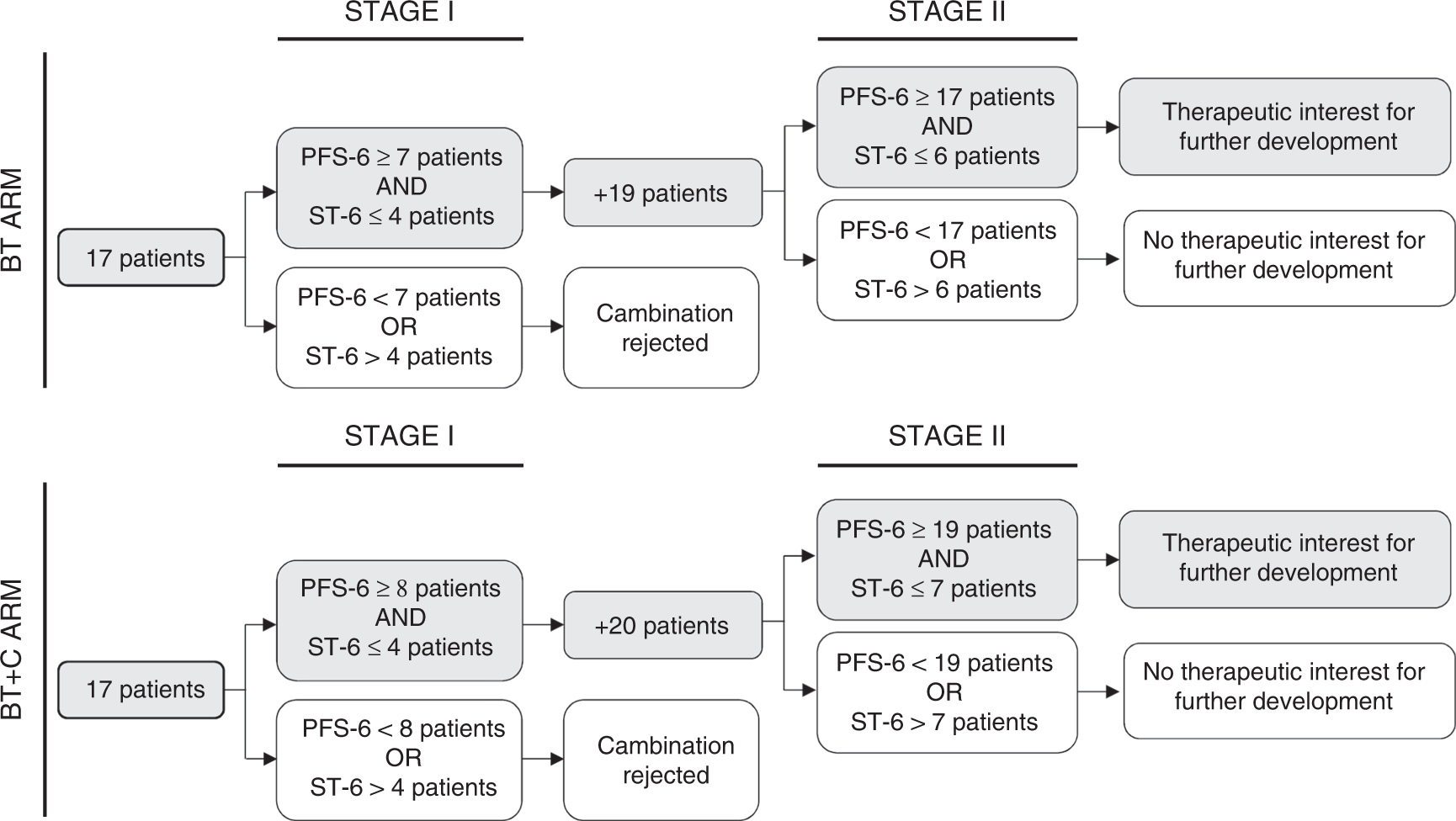
45 open-label study disadvantages
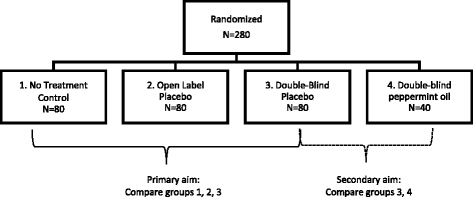
External and internal validity of open label or double-blind ... In these trials, open-label or double-blind double-dummy designs are being used to evaluate the efficacy and safety in prevention and treatment of venous thromboembolism or stroke prevention in atrial fibrillation in several thousands of patients. Bias for Patient-Reported Outcomes in Open-Label Cancer ... Feb 28, 2019 · A common concern with patient-reported outcomes (PROs) in open-label trials is that a patient’s knowledge of treatment received could influence their view and reporting of their symptoms. With this in mind, members of the US Food and Drug Administration explored the possibility of such bias in a recent viewpoint published in JAMA Oncology.
What is an Open-Label Clinical Trial? - News-Medical.net Clinical trials are vital for the design and development of safe drugs, treatments, and medical interventions and bringing them to market. Often, the idea for a clinical trial starts in the laboratory. Researchers will test new drugs and treatments in animal models, with the most promising being considered for clinical trials. There are four phases...
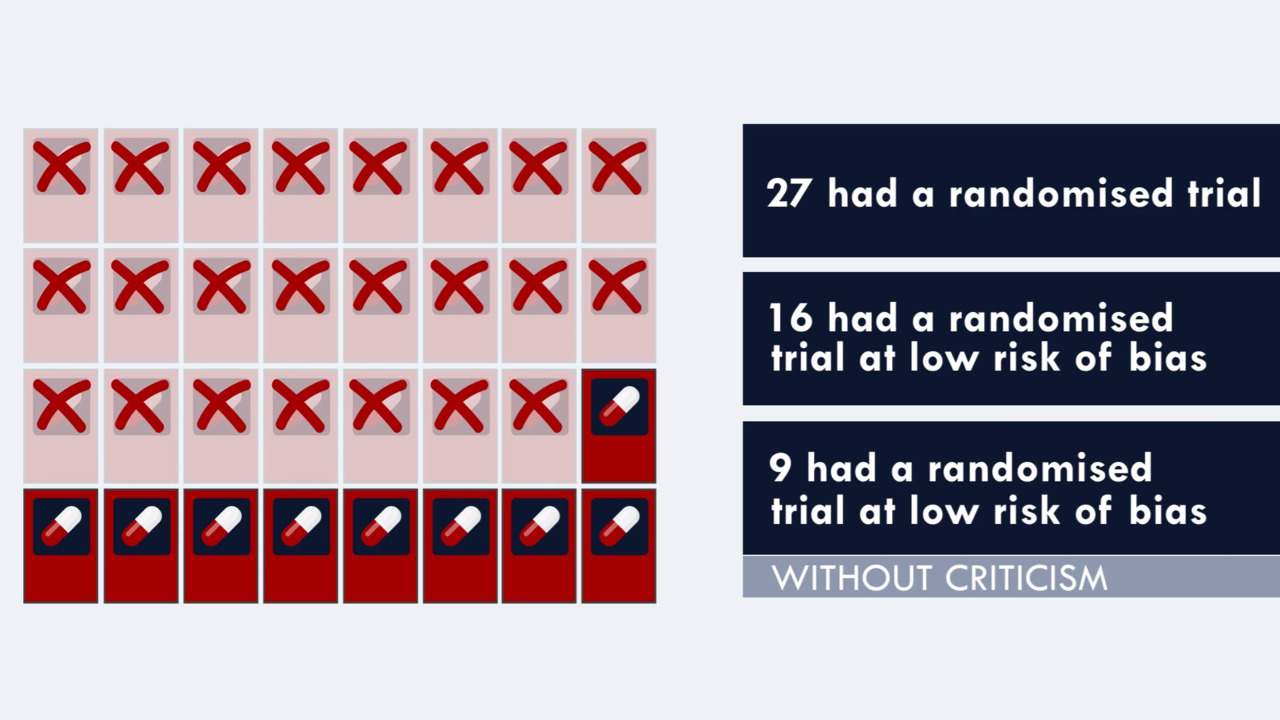
Open-label study disadvantages
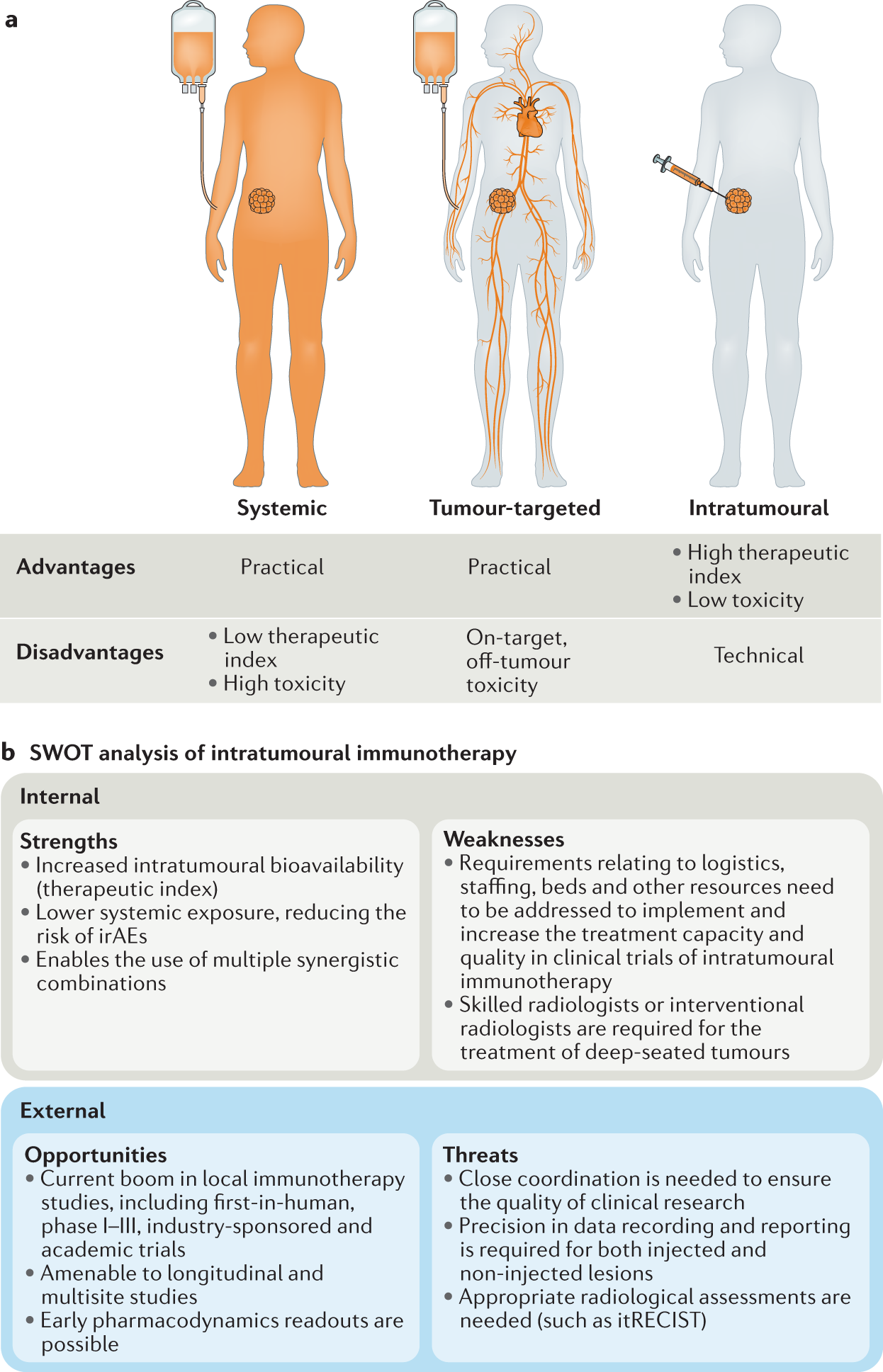
Statistical controversies in clinical research: limitations ... Apr 1, 2018 · These therapies are associated with substantial adverse effects, such as increased blood pressure, fistula formation, bowel perforation, and reversible posterior leuko-encephalopathy. A paradoxical vascular safety profile has been observed, as AA therapies have been associated with increased risks of both bleeding and thrombotic events [ 3, 4 ]. Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, [4] or possible relief from symptoms of some disorders when a placebo is given. [5] An open-label trial may still be randomized.
Open-label study disadvantages. Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, [4] or possible relief from symptoms of some disorders when a placebo is given. [5] An open-label trial may still be randomized. Statistical controversies in clinical research: limitations ... Apr 1, 2018 · These therapies are associated with substantial adverse effects, such as increased blood pressure, fistula formation, bowel perforation, and reversible posterior leuko-encephalopathy. A paradoxical vascular safety profile has been observed, as AA therapies have been associated with increased risks of both bleeding and thrombotic events [ 3, 4 ].

















:max_bytes(150000):strip_icc()/Vertical-integration-7a31b884b9564a139c5ec2f7885ff3f0.jpg)



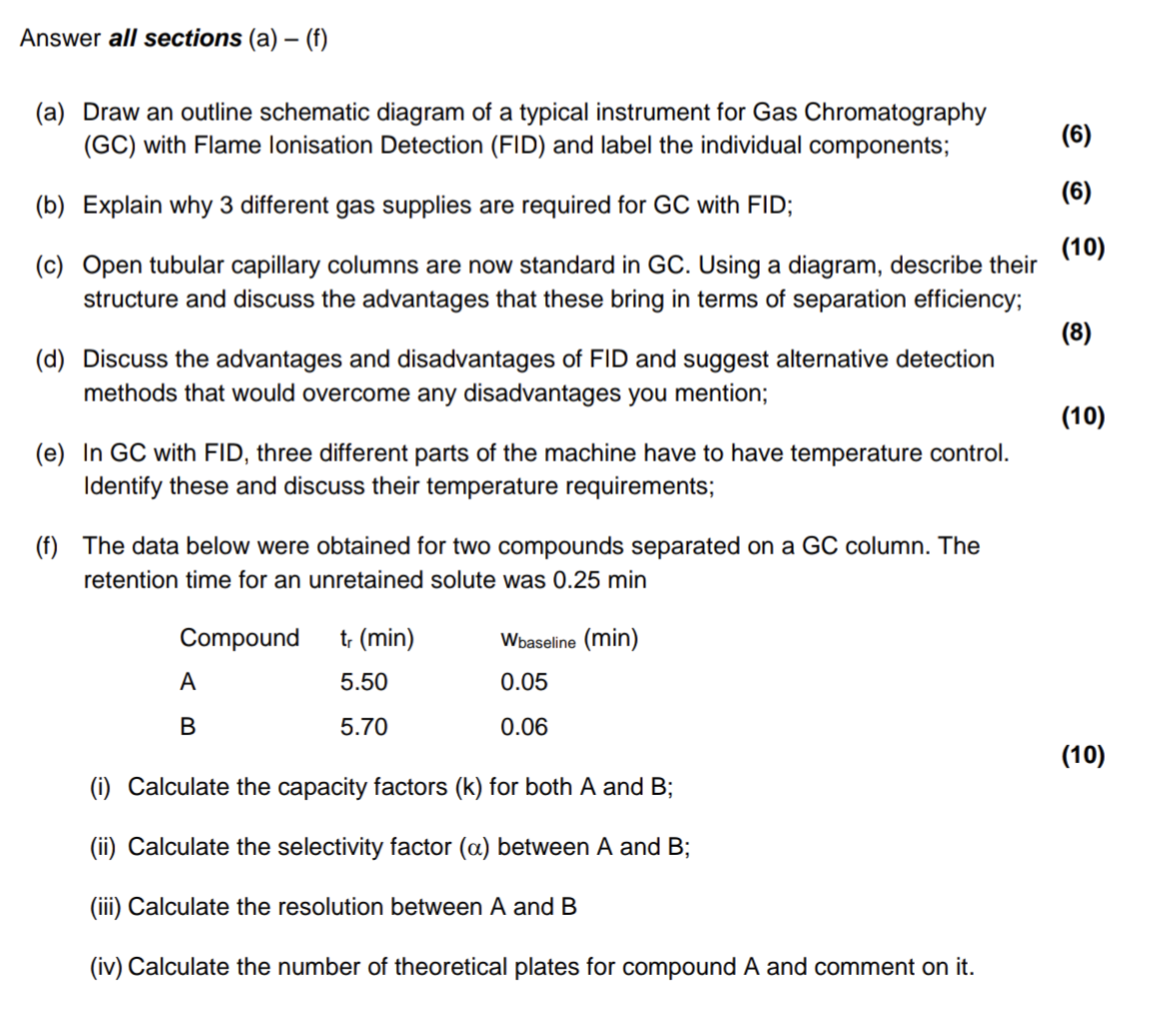
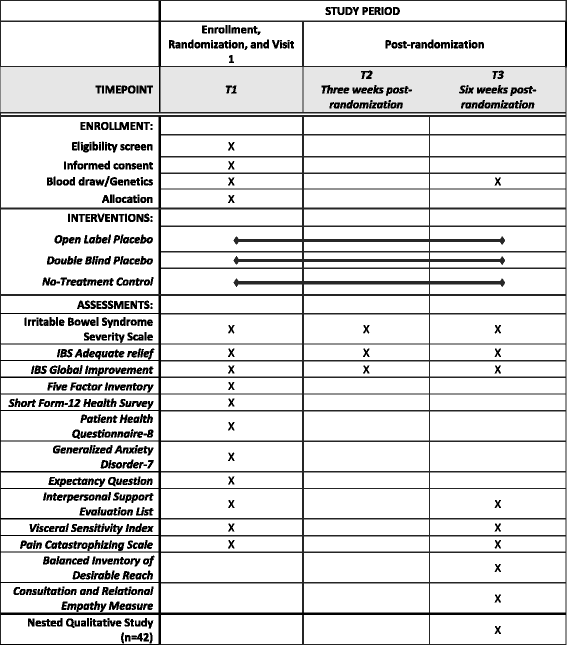
![PDF] Clinical trial methodology to assess the efficacy ...](https://d3i71xaburhd42.cloudfront.net/a00f5af9933a8f9c61da678199905782adf4210d/8-Table4-1.png)




Post a Comment for "45 open-label study disadvantages"